|
|
|
|
|
FMD | 前沿研究:基于系统功能基因组学注释解析肺腺癌与肺鳞癌的遗传异质性 |
|
|
论文标题:Comprehensive functional annotation of susceptibility variants identifies genetic heterogeneity between lung adenocarcinoma and squamous cell carcinoma (基于系统功能基因组学注释解析肺腺癌与肺鳞癌的遗传异质性)
期刊: Frontiers of Medicine
作者:Na Qin, Yuancheng Li, Cheng Wang, Meng Zhu, Juncheng Dai, Tongtong Hong, Demetrius Albanes, Stephen Lam, Adonina Tardon, Chu Chen, Gary Goodman, Stig E. Bojesen, Maria Teresa Landi, Mattias Johansson, Angela Risch, H-Erich Wichmann, Heike Bickeboller, Gadi Rennert, Susanne Arnold, Paul Brennan, John K. Field, Sanjay Shete, Loic Le Marchand, Olle Melander, Hans Brunnstrom, Geoffrey Liu, Rayjean J. Hung, Angeline Andrew, Lambertus A. Kiemeney, Shan Zienolddiny, Kjell Grankvist, Mikael Johansson, Neil Caporaso, Penella Woll, Philip Lazarus, Matthew B. Schabath, Melinda C. Aldrich, Victoria L. Stevens, Guangfu Jin, David C. Christiani, Zhibin Hu, Christopher I. Amos, Hongxia Ma, Hongbing Shen
发表时间:09 Sep 2020
DOI:10.1007/s11684-020-0779-4
微信链接:点击此处阅读微信文章
导读
南京医科大学沈洪兵院士团队在Frontiers of Medicine发表研究论文《基于系统功能基因组学注释解析肺腺癌与肺鳞癌的遗传异质性》(Comprehensive functional annotation of susceptibility variants identifies genetic heterogeneity between lung adenocarcinoma and squamous cell carcinoma)。南京医科大学秦娜博士后和李远程博士为本研究的共同第一作者,沈洪兵院士和马红霞教授为本研究的共同通讯作者。
非小细胞肺癌是全球最常见的恶性肿瘤之一。腺癌和鳞癌是其最主要的两种类型,具有明显的遗传多样性和病理组织异质性。由于肺癌的生物学特性复杂,恶性程度高,加强不同病理亚型肺癌的病因学和发病机制研究,识别高危人群进行精准预防是当前肺癌防治的迫切需求。
遗传因素是肺癌发生的主要危险因素之一。为了筛选特异的遗传标志物用于肺癌高危人群筛查,沈洪兵院士团队和国内外学者在亚洲和欧美人群中开展了一系列肺癌全基因组关联研究(GWAS),共鉴定到位于51个易感区域的81个易感变异。但是,目前易感变异的生物学功能解析仍面临一定的挑战,包括:易感变异仅为标签变异,致病变异未知;易感变异多位于基因组非编码区域,其生物学功能未知;易感区域致病基因未知等。因此,如何明确已知易感区域的致病变异和基因是“后GWAS”时期亟需解决的科学问题。
肿瘤的发生涉及到复杂的调控系统,需要整合多个层面的组学数据进行分析。近年来,生物学技术的快速发展为解析遗传变异-基因-疾病的关联提供了强有力的工具。因此,针对上述问题,本研究综合运用包括基因组、转录组和功能基因组等多种分子层面和来源的高通量组学数据和生物信息学算法,首次建立肺癌易感变异和易感基因致病性的功能评价体系,并明确了相关遗传变异和基因的生物学功能(图1)。该研究结果一方面有助于解析不同病理亚型肺癌发病相关的分子生物学机制,为后续功能学研究提供线索;另一方面,该研究鉴定出的致病变异将有助于肺癌的风险预测和诊断,实现从遗传学研究到临床应用的转化。
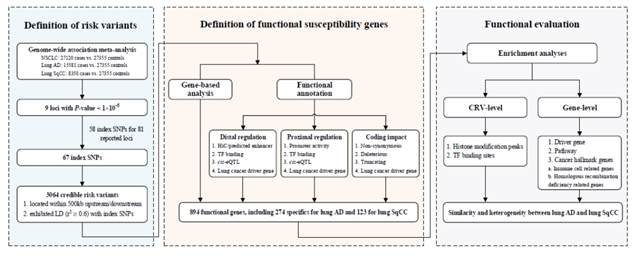
图1 研究设计流程图
摘要
全基因组关联研究已鉴定出一系列肺癌的遗传易感变异,然而这些变异的生物学功能尚不明确。为了系统解析肺癌易感变异的功能并揭示其影响肺癌发生的潜在易感机制,该研究首先基于27 120例非小细胞肺癌和27 355例健康对照的基因型数据进行荟萃分析,筛选肺癌易感性相关的遗传变异;随后,获取GTEx、TCGA、ENCODE、Roadmap和FANTOM5等数据库的基因组、转录组和表观基因组数据,并整合六种生物信息学预测算法,分别对非编码区和编码区的遗传变异进行功能评价。
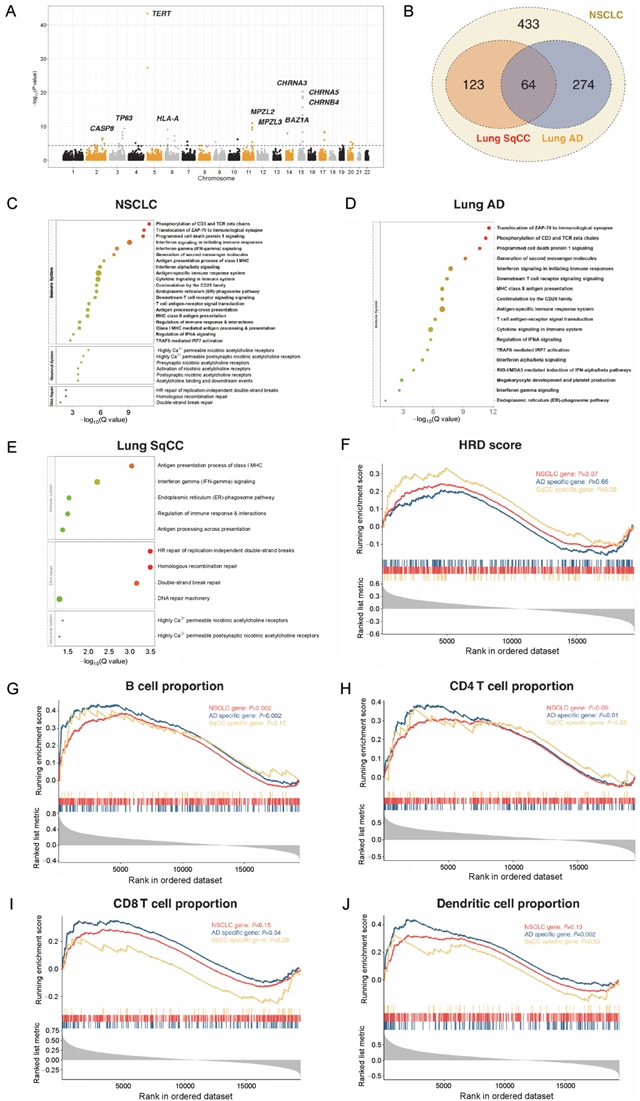
该研究共纳入3064个易感变异进行分析,其中,39个(1.27%)位于基因的编码区域;3025个位于非编码区域,且在启动子和增强子相关的组蛋白修饰区域以及DNase I超敏位点区域存在显著富集。组织病理学亚型特异的转录因子富集分析结果显示,肺腺癌特有的易感变异在转录因子USF1结合区域富集,而肺鳞癌特有的易感变异则在CREB1结合区域富集。变异的功能注释共定位到803个肺癌的潜在致病基因,包括274个肺腺癌特异和123个肺鳞癌特异的基因,提示两者的遗传易感机制存在区别。进一步的通路富集分析结果显示,肺腺癌和鳞癌共有的易感基因在尼古丁胆碱受体相关的通路富集,而腺癌和鳞癌特异的易感基因则分别在免疫和同源重组修复相关的通路富集。
该研究通过整合大样本人群的GWAS数据和多个分子水平的功能基因组学数据,首次建立了肺癌易感变异和基因的功能评价体系,并揭示了尼古丁成瘾、免疫炎症和DNA损伤在肺癌发生中的重要作用。该结果不仅从易感性的角度阐释了不同组织病理学亚型肺癌的发生机制,为肺癌的分子生物学研究提供新的方向,也为指导肺癌高危人群筛选和个体化预防提供了可靠的变异列表。
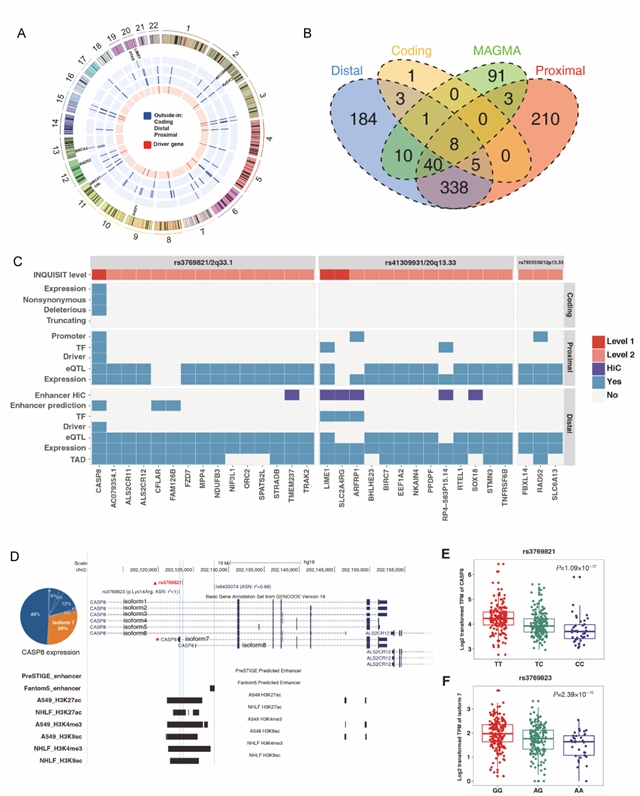
点评
该研究突破了既往遗传易感性研究的传统思路,综合运用流行病学、基因组学和功能基因组学数据以及生物信息学分析方法,首次建立肺癌易感变异和易感基因致病性的功能评价体系,对肺癌的发病机制进行探讨。该研究为后续肺癌遗传学病因研究提供新的研究思路,也为解释肺癌的发生机制、筛查高危人群提供了全新的视角。
原文信息
标题
Comprehensive functional annotation of susceptibility variants identifies genetic heterogeneity between lung adenocarcinoma and squamous cell carcinoma
作者
Na Qin, Yuancheng Li, Cheng Wang, Meng Zhu, Juncheng Dai, Tongtong Hong, Demetrius Albanes, Stephen Lam, Adonina Tardon, Chu Chen, Gary Goodman, Stig E. Bojesen, Maria Teresa Landi, Mattias Johansson, Angela Risch, H-Erich Wichmann, Heike Bickeboller, Gadi Rennert, Susanne Arnold, Paul Brennan, John K. Field, Sanjay Shete, Loic Le Marchand, Olle Melander, Hans Brunnstrom, Geoffrey Liu, Rayjean J. Hung, Angeline Andrew, Lambertus A. Kiemeney, Shan Zienolddiny, Kjell Grankvist, Mikael Johansson, Neil Caporaso, Penella Woll, Philip Lazarus, Matthew B. Schabath, Melinda C. Aldrich, Victoria L. Stevens, Guangfu Jin, David C. Christiani, Zhibin Hu, Christopher I. Amos, Hongxia Ma, Hongbing Shen
机构
1. Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing 211166, China
2. State Key Laboratory of Reproductive Medicine, Center for Global Health, Nanjing Medical University, Nanjing 211166, China
3. Department of Bioinformatics, School of Biomedical Engineering and Informatics, Nanjing Medical University, Nanjing 211166, China
4. Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Medicine, Nanjing Medical University, Nanjing 211166, China
5. China International Cooperation Center for Environment and Human Health, School of Public Health, Nanjing Medical University, Nanjing 211166, China
6. Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD 20892-9304, USA
7. Department of Integrative Oncology, British Columbia Cancer Agency, Vancouver, BC V5Z 1L3, Canada
8. Faculty of Medicine, University of Oviedo and CIBERESP, Oviedo 33006, Spain
9. Program in Epidemiology, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA 98109-1024, USA
10. Public Health Sciences Division, Swedish Cancer Institute, Seattle, WA 98026, USA
11. Department of Clinical Biochemistry, Copenhagen University Hospital, Copenhagen DK-1017, Denmark
12. National Cancer Institute, Bethesda, MD 20892-9304, USA
13. Genetic Epidemiology Group, International Agency for Research on Cancer, Lyon 69372, France
14. Cancer Center Cluster Salzburg at PLUS, Department of Molecular Biology, University of Salzburg, Heidelberg 5020, Austria
15. Institute of Medical Informatics, Biometry and Epidemiology, Chair of Epidemiology, Ludwig Maximilians University, Munich, Bavaria 80539, Germany
16. Department of Genetic Epidemiology, University Medical Center Goettingen, Goettingen 37075, Germany
17. Technion Faculty of Medicine, Carmel Medical Center, Haifa 3448516, Israel
18. Markey Cancer Center, University of Kentucky, Lexington, KY 40506-0054, USA
19. Department of Molecular and Clinical Cancer Medicine, Roy Castle Lung Cancer Research Programme, The University of Liverpool Institute of Translational Medicine, Liverpool L69 7ZX, UK
20. Department of Epidemiology, The University of Texas, MD Anderson Cancer Center, Houston, TX 77079, USA
21. Department of Epidemiology, University of Hawaii Cancer Center, Honolulu, HI 96813, USA
22. Department of Clinical Sciences, Lund University, BMC F12, 221 84, Sweden
23. Epidemiology Division, Princess Margaret Cancer Center, Toronto, ON M4Y 2H8, Canada
24. Epidemiology Division, Lunenfeld-Tanenbuaum Research Institute, Sinai Health System, Toronto, ON M5G 1X5, Canada
25. Department of Neurology, Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756, USA
26. Department of Health Evidence, Radboud University Medical Center, Nijmegen 9101 6500 HB, Germany
27. National Institute of Occupational Health (STAMI), Oslo Pb 5330, Norway
28. Department of Medical Biosciences, Umeå University, Umea 901 87, Sweden
29. Department of Radiation Sciences, Umeå University, Umea 901 87, Sweden
30. Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD 20850, USA
31. Academic Unit of Clinical Oncology, University of Sheffield, Sheffield S10 2TN, UK
32. College of Pharmacy, Washington State University, Spokane, WA 99210, USA
33. Department of Cancer Epidemiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL 12902, USA
34. Department of Thoracic Surgery, Division of Epidemiology, Vanderbilt University Medical Center, Nashville, TN 37232, USA
35. Department of Epidemiology Research Program, American Cancer Society, Atlanta, GA 30303, USA
36. Department of Environmental Health, Harvard School of Public Health, Department of Medicine, Harvard Medical School/Massachusetts General Hospital, Boston, MA 02115, USA
37. Baylor College of Medicine, Institute for Clinical and Translational Research, Houston, TX 21202, USA
Corresponding Authors Hongxia Ma and Hongbing Shen
Cite this article
Na Qin, Yuancheng Li, Cheng Wang, Meng Zhu, Juncheng Dai, Tongtong Hong, Demetrius Albanes, Stephen Lam, Adonina Tardon, Chu Chen, Gary Goodman, Stig E. Bojesen, Maria Teresa Landi, Mattias Johansson, Angela Risch, H-Erich Wichmann, Heike Bickeboller, Gadi Rennert, Susanne Arnold, Paul Brennan, John K. Field, Sanjay Shete, Loic Le Marchand, Olle Melander, Hans Brunnstrom, Geoffrey Liu, Rayjean J. Hung, Angeline Andrew, Lambertus A. Kiemeney, Shan Zienolddiny, Kjell Grankvist, Mikael Johansson, Neil Caporaso, Penella Woll, Philip Lazarus, Matthew B. Schabath, Melinda C. Aldrich, Victoria L. Stevens, Guangfu Jin, David C. Christiani, Zhibin Hu, Christopher I. Amos, Hongxia Ma, Hongbing Shen. Comprehensive functional annotation of susceptibility variants identifies genetic heterogeneity between lung adenocarcinoma and squamous cell carcinoma. Front. Med., 2021, 15(2): 275–291 https://doi.org/10.1007/s11684-020-0779-4
https://journal.hep.com.cn/fmd/EN/10.1007/s11684-020-0779-4
https://link.springer.com/article/10.1007/s11684-020-0779-4
摘要
Although genome-wide association studies have identified more than eighty genetic variants associated with non-small cell lung cancer (NSCLC) risk, biological mechanisms of these variants remain largely unknown. By integrating a large-scale genotype data of 15 581 lung adenocarcinoma (AD) cases, 8350 squamous cell carcinoma (SqCC) cases, and 27 355 controls, as well as multiple transcriptome and epigenomic databases, we conducted histology-specific meta-analyses and functional annotations of both reported and novel susceptibility variants. We identified 3064 credible risk variants for NSCLC, which were overrepresented in enhancer-like and promoter-like histone modification peaks as well as DNase I hypersensitive sites. Transcription factor enrichment analysis revealed that USF1 was AD-specific while CREB1 was SqCC-specific. Functional annotation and gene-based analysis implicated 894 target genes, including 274 specifics for AD and 123 for SqCC, which were overrepresented in somatic driver genes (ER=1.95, P=0.005). Pathway enrichment analysis and Gene-Set Enrichment Analysis revealed that AD genes were primarily involved in immune-related pathways, while SqCC genes were homologous recombination deficiency related. Our results illustrate the molecular basis of both well-studied and new susceptibility loci of NSCLC, providing not only novel insights into the genetic heterogeneity between AD and SqCC but also a set of plausible gene targets for post-GWAS functional experiments.
点击左下角蓝色“阅读原文”, 可下载全文,了解更多内容。 感谢作者对Frontiers of Medicine的信任和支持。

《前沿》系列英文学术期刊
由教育部主管、高等教育出版社主办的《前沿》(Frontiers)系列英文学术期刊,于2006年正式创刊,以网络版和印刷版向全球发行。系列期刊包括基础科学、生命科学、工程技术和人文社会科学四个主题,是我国覆盖学科最广泛的英文学术期刊群,其中13种被SCI收录,其他也被A&HCI、Ei、MEDLINE或相应学科国际权威检索系统收录,具有一定的国际学术影响力。系列期刊采用在线优先出版方式,保证文章以最快速度发表。
中国学术前沿期刊网
http://journal.hep.com.cn

特别声明:本文转载仅仅是出于传播信息的需要,并不意味着代表本网站观点或证实其内容的真实性;如其他媒体、网站或个人从本网站转载使用,须保留本网站注明的“来源”,并自负版权等法律责任;作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。