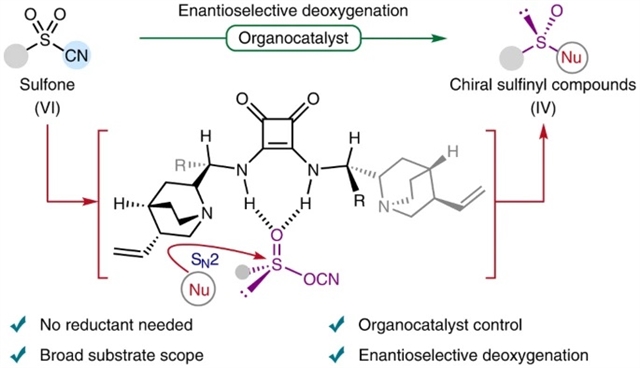
重庆大学闫海龙团队报道了砜的有机催化不对称脱氧制备手性亚磺酰基化合物。相关研究成果发表在2023年1月16日出版的《自然—化学》。
在过去的几十年中,已经开发了许多有效的方法实现手性亚磺酰基化合物的对映选择性合成。然而,六价砜的对映选择性脱氧以形成手性亚磺酰基化合物仍然是不对称合成和有机硫化学领域的主要挑战之一。
该文中,研究发现有机催化和氰基结合到砜中的协同组合生成了作为活性中间体手性亚磺酸物种。然后,可以使用醇作为亲核试剂获得具有高对映选择性的广泛手性亚磺酸酯,随后的转化能够集体制备各种手性亚磺酰基化合物。密度泛函理论计算表明,催化循环包括奎宁环辅助的逐步1,2-氰基转移、碱辅助的醇分子间取代和活性催化剂的再生。通过氰基迁移步骤确定对映体选择性。
附:英文原文
Title: Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
Author: Huang, Shengli, Zeng, Zhen, Zhang, Nan, Qin, Wenling, Lan, Yu, Yan, Hailong
Issue&Volume: 2023-01-16
Abstract: Over the past decades, many efficient methodologies have been developed that allow for the enantioselective synthesis of chiral sulfinyl compounds. However, the enantioselective deoxygenation of hexavalent sulfones for the formation of chiral sulfinyl compounds still remains one of the major challenges in the fields of asymmetric synthesis and organosulfur chemistry. Here we have demonstrated that a synergistic combination of organocatalysis and the incorporation of a cyano group into the sulfone generates a chiral sulfinic species as an active intermediate. A wide range of chiral sulfinates with high enantioselectivities could then be acquired using alcohols as nucleophiles, and the subsequent transformations allowed the collective preparation of a variety of chiral sulfinyl compounds. Density functional theory calculations revealed that the catalytic cycle involves a quinuclidine-assisted stepwise 1,2-cyano group transfer, base-assisted intermolecular substitution with alcohol and regeneration of the active catalyst. The enantioselectivity was determined by the cyano migration step.
DOI: 10.1038/s41557-022-01120-x
Source: https://www.nature.com/articles/s41557-022-01120-x
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
