荷兰乌得勒支大学Friedrich Förster
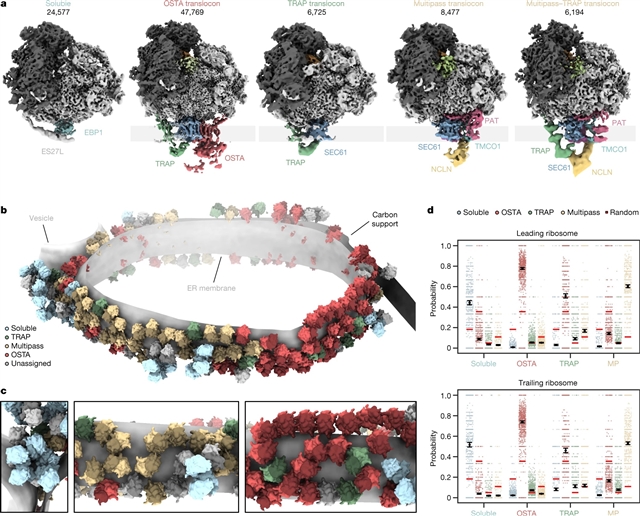
研究人员使用低温电子断层扫描、广泛的分类和分子建模以分子分辨率捕获了ER膜上mRNA翻译和蛋白质成熟的快照。研究人员发现了一个高度丰富的带有真核延伸因子1a(eEF1a)的经典易位前中间体,其处于扩展构象,这表明eEF1a可能在GTP水解后仍与核糖体结合。在内质网膜上,不同的多聚核糖体结合到不同的内质网转位子上,这些转位子专门合成带有信号肽的蛋白质或多通道跨膜蛋白,两种蛋白中都存在转位子相关蛋白复合物(TRAP)。
最丰富的ER转位子变体的近完整原子模型包括蛋白质传导通道SEC61、TRAP和低聚糖转移酶复合物A(OSTA),这揭示了TRAP与其他转位子组分的特定相互作用。研究人员观察到与OSTA相关的化学计量学和亚化学计量学辅助因子,其中可能包括蛋白质异构酶。总之,研究人员可视化了ER结合的多聚核糖体及其协调的下游机制。
据介绍,动态核糖体-转位子复合体位于ER膜,产生人类蛋白质组的主要部分。它控制新生蛋白质的合成、易位、膜插入、N-糖基化、折叠和二硫键的形成。虽然这一机器的各个组成部分已经在高分辨率下进行了单独研究,但对它们在原生膜中相互作用的见解仍然有限。
附:英文原文
Title: Visualization of translation and protein biogenesis at the ER membrane
Author: Gemmer, Max, Chaillet, Marten L., van Loenhout, Joyce, Cuevas Arenas, Rodrigo, Vismpas, Dimitrios, Grllers-Mulderij, Mariska, Koh, Fujiet A., Albanese, Pascal, Scheltema, Richard A., Howes, Stuart C., Kotecha, Abhay, Fedry, Juliette, Frster, Friedrich
Issue&Volume: 2023-01-25
Abstract: The dynamic ribosome–translocon complex, which resides at the endoplasmic reticulum (ER) membrane, produces a major fraction of the human proteome1,2. It governs the synthesis, translocation, membrane insertion, N-glycosylation, folding and disulfide-bond formation of nascent proteins. Although individual components of this machinery have been studied at high resolution in isolation3,4,5,6,7, insights into their interplay in the native membrane remain limited. Here we use cryo-electron tomography, extensive classification and molecular modelling to capture snapshots of mRNA translation and protein maturation at the ER membrane at molecular resolution. We identify a highly abundant classical pre-translocation intermediate with eukaryotic elongation factor 1a (eEF1a) in an extended conformation, suggesting that eEF1a may remain associated with the ribosome after GTP hydrolysis during proofreading. At the ER membrane, distinct polysomes bind to different ER translocons specialized in the synthesis of proteins with signal peptides or multipass transmembrane proteins with the translocon-associated protein complex (TRAP) present in both. The near-complete atomic model of the most abundant ER translocon variant comprising the protein-conducting channel SEC61, TRAP and the oligosaccharyltransferase complex A (OSTA) reveals specific interactions of TRAP with other translocon components. We observe stoichiometric and sub-stoichiometric cofactors associated with OSTA, which are likely to include protein isomerases. In sum, we visualize ER-bound polysomes with their coordinated downstream machinery.
DOI: 10.1038/s41586-022-05638-5
Source: https://www.nature.com/articles/s41586-022-05638-5
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
