瑞士苏黎世联邦理工学院Arosio, Paolo团队报道了RNA调节生物分子凝聚物介导的hnRNPA1A淀粉样蛋白形成。相研究成果于2024年3月12日发表在国际顶尖学术期刊《自然—化学》。
参与无膜细胞器的几种RNA结合蛋白,可以形成与神经退行性疾病相关的病理性淀粉样蛋白,但这种聚集是如何调节的机制仍然难以捉摸。
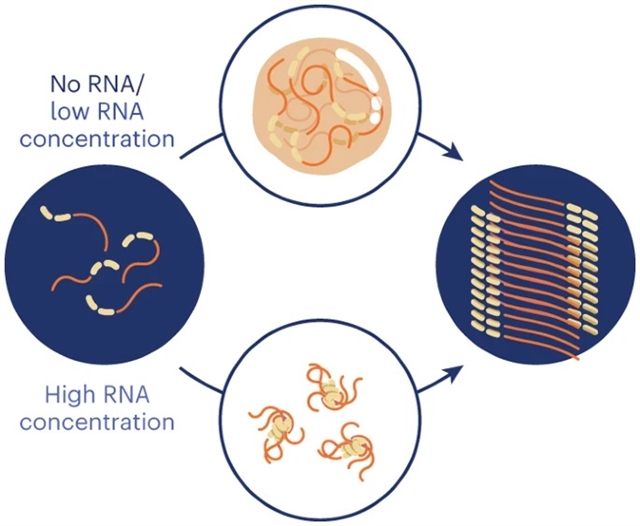
该文中,研究人员研究了异型蛋白-RNA相互作用如何调节hnRNPA1A的凝结,和液体向淀粉样蛋白的转变,hnRNPA1A是一种参与嗜淀粉性侧索硬化的蛋白质。在不存在RNA的情况下,缩合物的形成促进hnRNPA1A的聚集,并且原纤维定位在缩合物的界面处。
根据RNA/蛋白质化学计量的不同途径,添加RNA调节hnRNPA1A的可溶性向淀粉样蛋白的转变。在低RNA浓度下,RNA促进缩合和淀粉样蛋白的形成,RNA的催化作用增加了浓相和稀相之间界面的作用。在较高的RNA浓度下,根据再入相行为,缩合被抑制,但在较长的孵育时间内观察到hnRNPA1A淀粉样蛋白的形成。
研究结果显示了异型核酸-蛋白质相互作用,如何影响淀粉样蛋白形成的动力学和分子途径。
附:英文原文
Title: RNA modulates hnRNPA1A amyloid formation mediated by biomolecular condensates
Author: Morelli, Chiara, Faltova, Lenka, Capasso Palmiero, Umberto, Makasewicz, Katarzyna, Papp, Marcell, Jacquat, Raphal P. B., Pinotsi, Dorothea, Arosio, Paolo
Issue&Volume: 2024-03-12
Abstract: Several RNA binding proteins involved in membraneless organelles can form pathological amyloids associated with neurodegenerative diseases, but the mechanisms of how this aggregation is modulated remain elusive. Here we investigate how heterotypic protein–RNA interactions modulate the condensation and the liquid to amyloid transition of hnRNPA1A, a protein involved in amyothropic lateral sclerosis. In the absence of RNA, formation of condensates promotes hnRNPA1A aggregation and fibrils are localized at the interface of the condensates. Addition of RNA modulates the soluble to amyloid transition of hnRNPA1A according to different pathways depending on RNA/protein stoichiometry. At low RNA concentrations, RNA promotes both condensation and amyloid formation, and the catalytic effect of RNA adds to the role of the interface between the dense and dilute phases. At higher RNA concentrations, condensation is suppressed according to re-entrant phase behaviour but formation of hnRNPA1A amyloids is observed over longer incubation times. Our findings show how heterotypic nucleic acid–protein interactions affect the kinetics and molecular pathways of amyloid formation.
DOI: 10.1038/s41557-024-01467-3
Source: https://www.nature.com/articles/s41557-024-01467-3
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
